



We provide our biotech and pharmaceutical partners with fully integrated services to support their efforts from target identification through to commercial drug product manufacturing. Download this flyer to see the breadth of our services.

With focused centers of excellence in the United States and China, BioDuro has the flexibility and breadth to engage with you at any level of the drug development and manufacturing process.

Comprehensive and readily available drug metabolism identification, profiling, stability and inhibition services with extensive capabilities in Cytochrome P450 (CYP) and UDP-glucuronosyltransferases (UGT).

With as low as milligram quantities of API, we can provide physical, chemical and biological characterization data to drive quick and sound decisions on the best formulation approach for late discovery and preclinical phases through the development of human clinical trial material.

Our analytical development team is well-versed to facilitate the development of the analytical methods and testing of prototype formulations, raw materials, and drug products spanning from Pre-IND early phase to late registration.

View our recent IND Enabling Services case study of an 11 month timeline to IND ready. Learn about our API process development and drug product formulation alongside both non-GMP and GMP production activities, and the final CTD documents.
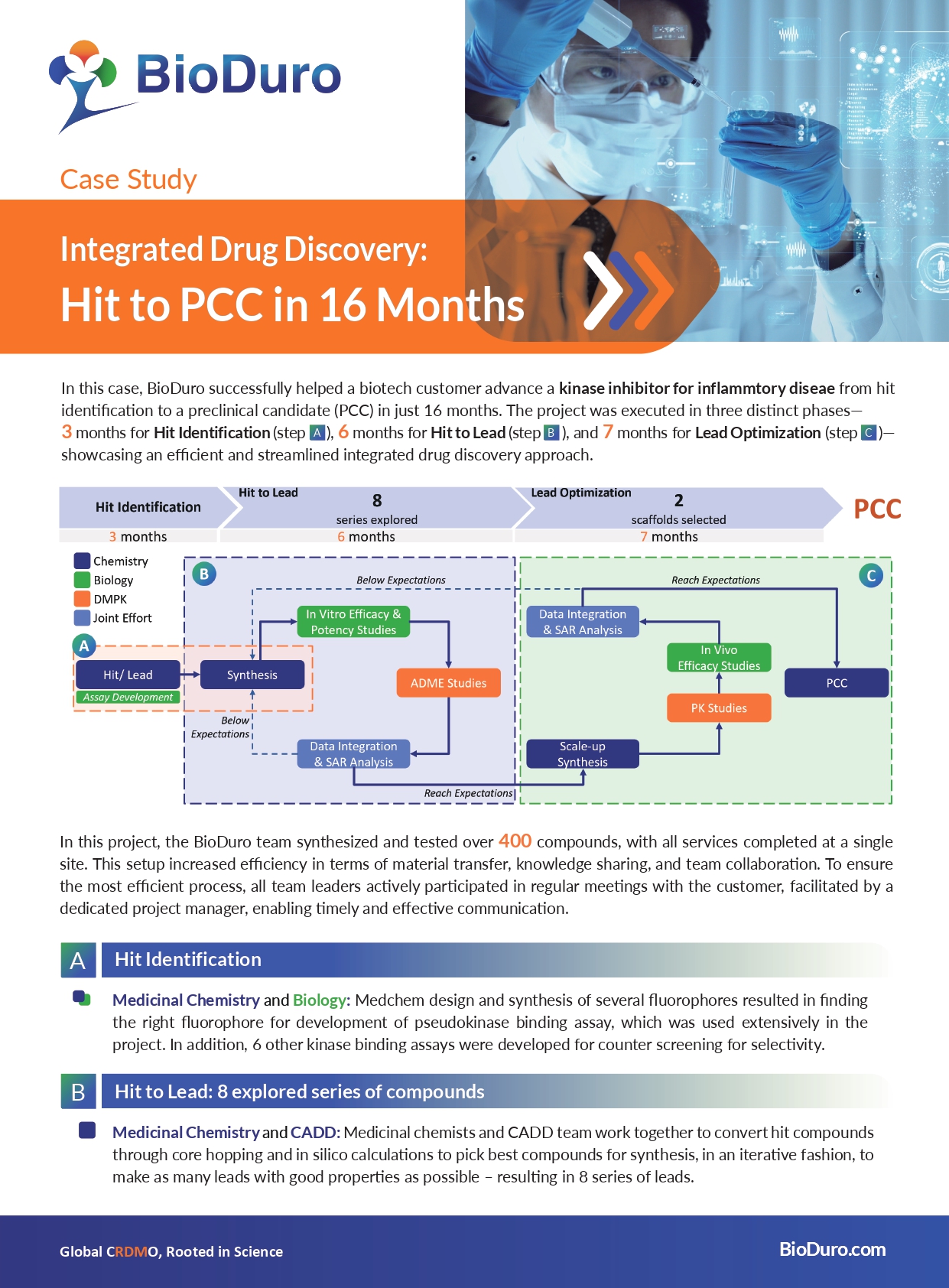
Our uniquely integrated drug discovery platform advanced a biotech customer's kinase inhibitor for inflammatory disease from hit identification to a preclinical candidate (PCC) in just 16 months.

A full list of all our available CDX Models includes: bladder cancer, blood cancer, brain cancer, breast cancer, cervix cancer, choriocarcinoma, colorectal cancer, esophageal cancer, fibrosarcoma, gastric cancer, kidney cancer, and more.

Learn about our automated, flexible tailored solutions to store and manage compounds in both liquid and solid formats. Our compound management platform includes the advanced Hamilton Liquid Handling System and end-to-end Mosaic System, providing accurate and efficient oversight of our compound inventory.

Discover our integrated drug discovery solutions with a wide range of cancer research models, immuno-oncology techniques, and comprehensive cellular and biomarker analyses. Our services include drug target discovery, potency and efficacy evaluation, and access to the LOCUS Queryable Database to identify oncology models.

Partner with us for your drug discovery project needs. We provide a wide range of services, including custom assays, stable cell line generation, SPR and BLI services, and fragment library screening, with broad expertise in therapeutic disease areas and target class.

Partner with our experienced and specialized team to solve complex chemistry problems. As a service-oriented business, we offer long-term partnerships and tailored solutions. We will help you to achieve your chemistry goals.

Fast and reliable DMPK services covering in vitro and in vivo assays to evaluate ADME and PK properties, and subsequent preclinical services for a wide range of modalities.

Our comprehensive range of services, from early drug discovery to commercial API production, includes drug substance development, process R&D, scale-up synthesis, and integrated IND enabling services. Our expert team is dedicated to ensuring quality and efficiency to help you bring new drugs to market quickly and effectively.

Offering a diverse and efficient fragment library with novel scaffolds and important pharmacophores, excluding functional groups with known structural alerts, for drug discovery projects. Learn more about our screening and hit follow-up options.

BioDuro in vitro and in vivo drug discovery services address preclinical research needs in the development of pharmaceuticals to treat immune, autoimmune, and inflammatory diseases.


We leverage decades of experience and expertise in peptide chemistry to deliver high-quality peptide, from milligram to gram scale. Our state-of-art facilities, including a dedicated 1500 ㎡ peptide synthesis center in Beijing, are equipped with top-class instruments and supported by a team of 100+ experienced chemists.

Fast and flexible in vivo PK services with 10 species of rodents, small and large animals, and a range of advanced administration and bioanalysis techniques.

A case study on: Formulation Development of Amorphous Solid Dispersions by Combined In-Silico Modelling, High-Throughput Screening and in-vivo PK Study.