Common Technical Document (CTD)
The Common Technical Document (CTD) is a standard format for presenting data in an Investigational New Drug (IND) application to the FDA, ensuring consistent and comprehensive information for regulatory review.
The CTD consists of quality, nonclinical and clinical attributes of required IND documentation.
CTD Triangle
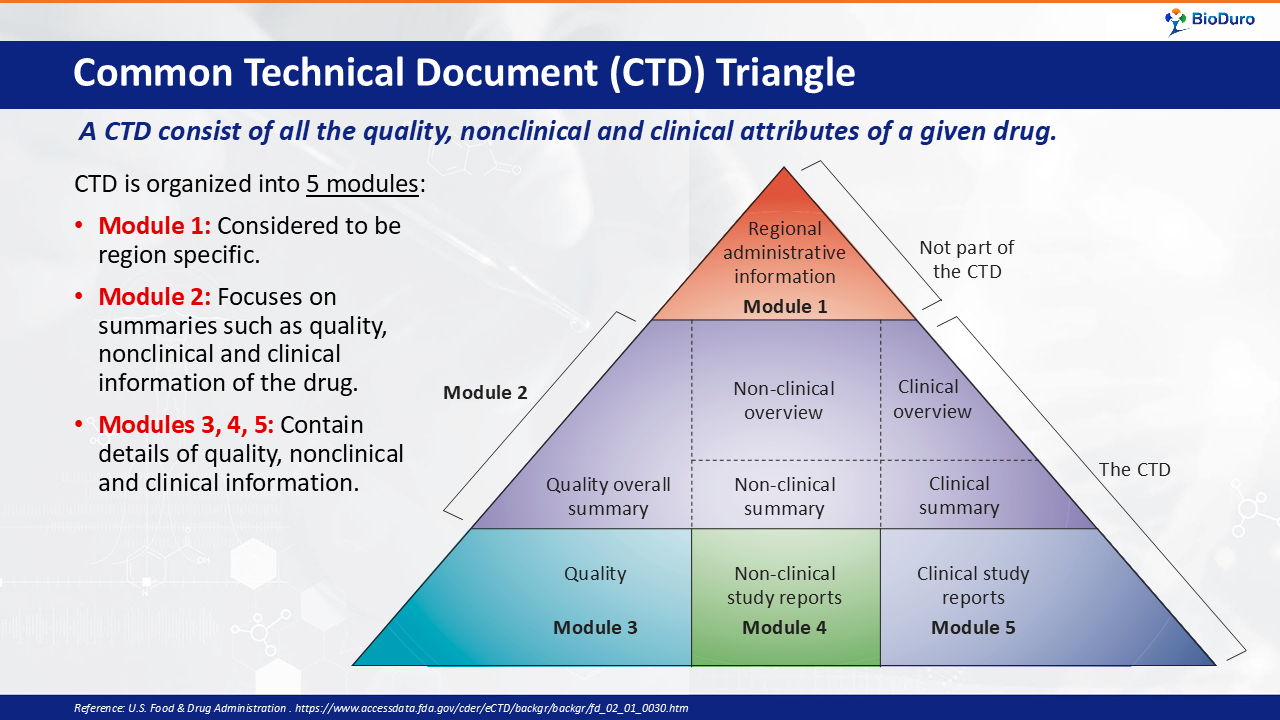
What is the CTD Triangle? The Common Technical Document (CTD) can be represented by a visual triangle or "pyramid" of the required documents which must be organized and complete before submitting a new investigational drug (IND) application to the FDA.
Module 1, at the peak of the pyramid, contains regional administrative information.
Module 2, mid-tier in the pyramid, focuses on summaries such as quality, nonclinical and clinical information for the investigational new drug (IND).
Module 3, at the base of the pyramid, contains the details of quality, nonclinical and clinical information.
BioDuro-Sundia CTD and IND Services
Our dedicated regulatory team provides comprehensive services including the CTD in full support of our clients' IND applications to the FDA, NMPA, and other respected regulatory agencies with bilingual translation available.
We support all aspects client IND applications, including dossier, data and communication. In addition to the complete Common Technical Cocument (CTD) management, BioDuro-Sundia performs a comprehensive range of preclinical in vitro and in vivo studies needed to prepare for an IND application.
Learn more about our IND Enabling platform.FAQ: IND Filing and Documentation Services
Q: Does BioDuro-Sundia have a regulatory team capable providing support and communication with the FDA?
A: Yes, our dedicated regulatory team regularly communicates with the US FDA on behalf of clients with IND applications in progress. With years of experience, our experts provide comprehensive support, including direct communication with the FDA, addressing inquiries, and preparing supplemental documentation. This ensures a smooth and successful filing process for our clients.
Q: To what extent does BioDuro-Sundia put the IND application materials together, for example, can your CRDMO submit INDs to both the US and China regulatory agencies?
A: Yes, our regulatory team is fully equipped to prepare comprehensive IND application materials for our clients, covering both scientific and regulatory aspects. In addition to our preclinical CMC services, we provide integrated DMPK, toxicology, and bioanalysis support to ensure a seamless IND submission process. While the majority of our clients submit to the U.S. FDA, we are also experienced in preparing dual submissions for both the U.S. FDA and China’s NMPA. We tailor the documentation to meet the distinct regulatory requirements and formatting guidelines of each agency, enabling parallel submissions in English and Chinese as needed.
Q: Can BioDuro-Sundia support all IND activities in-house?
A: Yes, we perform all preclinical IND-enabling activities in-house, and in some cases, everything can be carried out at a single facility to ensure optimal speed and efficiency. Our fully integrated approach covers all the essential services required for IND submission, including API and drug product R&D, DMPK, pharmacology, non-GMP and GMP manufacturing for both API and drug product, as well as analytical and quality control (QC) services, such as stability testing. These elements work seamlessly together to provide a streamlined and efficient process.
Q: Can you offer an example of a recent successful integrated IND program at BioDuro-Sundia?
A: Recently, we successfully supported a client with a complex small molecule IND program submitted to the U.S. FDA. The program involved an intricate 8-step process, during which our team managed both R&D and production for the API and drug product, as well as the preparation of the regulatory dossier. Despite the complexity, we completed the entire project—from initial development to submission—in just 11 months.
Read more